"Electronegativity" is antipodally distinguished from "Electropositivity," which describes an element's ability to donate electrons.
Electronegativity, symbol χ, is a chemical property that describes the power of an atom (or, more rarely, a functional group) to attract electrons towards itself. but it is usually considered to be a transferable property, that is to say that similar values will be valid in a variety of situations.
Electronegativities of the elements
 Methods of calculation
Methods of calculationPauling first proposed and it is these "revised Pauling" values of the electronegativity which are most usually used.
Pauling electronegativity
Mulliken proposed that the arithmetic mean of the first ionization energy and the electron affinity should be a measure of the tendency of an atom to attract electrons.

The Mulliken electronegativity can only be calculated for an element for which the electron affinity is known, fifty-seven elements as of 2006.
Allred-Rochow electronegativity
Sanderson has also noted the relationship between electronegativity and atomic size, and has proposed a method of calculation based on the reciprocal of the atomic volume.
Sanderson electronegativity
Perhaps the simplest definition of electronegativity is that of Allen, who has proposed that it is related to the average energy of the valence electrons in a free atom, However, it is not clear what should be considered to be valence electrons for the d- and f-block elements, which leads to an ambiguity for their electronegativities calculated by the Allen method.
Allen electronegativity
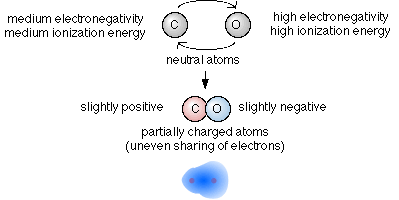
The wide variety of methods of calculation of electronegativities, which all give results which correlate well with one another, is one indication of the number of chemical properties which might be affected by electronegativity. The most obvious application of electronegativities is in the discussion of bond polarity, for which the concept was introduced by Pauling. In general, the greater the difference in electronegativity between two atoms, the more polar the bond that will be formed between them, with the atom having the higher electronegativity being at the negative end of the dipole. Pauling proposed an equation to relate "ionic character" of a bond to the difference in electronegativity of the two atoms,
Correlation of electronegativity with other properties
Trends in electronegativity
In general, electronegativity increases on passing from left to right along a period, and decreases on descending a group. Hence, fluorine is undoubtedly the most electronegative of the elements while caesium is the least electronegative, at least of those elements for which substantial data are available.
There are some exceptions to this general rule. Gallium and germanium have higher electronegativities than aluminium and silicon respectively because of the d-block contraction. Elements of the fourth period immediately after the first row of the transition metals have unusually small atomic radii because the 3d-electrons are not effective at shielding the increased nuclear charge, and smaller atomic size correlates with higher electronegativity (see Allred-Rochow electronegativity, Sanderson electronegativity above). The anomalously high electronegativity of lead, particularly when compared to thallium and bismuth, appears to be an artifact of data selection (and data availability)—methods of calculation other than the Pauling method show the normal periodic trends for these elements.
Periodic trends
In inorganic chemistry it is common to consider a single value of the electronegativity to be valid for most "normal" situations. While this approach has the advantage of simplicity, it is clear that the electronegativity of an element is not an invariable atomic property and, in particular, increases with the oxidation state of the element.
Allred used the Pauling method to calculate separate electronegativities for different oxidation states of the handful of elements (including tin and lead) for which sufficient data was available. However, for most elements, there are not enough different covalent compounds for which bond dissociation energies are known to make this approach feasible. This is particularly true of the transition elements, where quoted electronegativity values are usually, of necessity, averages over several different oxidation states and where trends in electronegativity are harder to see as a result.
The chemical effects of this increase in electronegativity can be seen both in the structures of oxides and halides and in the acidity of oxides and oxoacids. Hence CrO3 and Mn2O7 are acidic oxides with low melting points, while Cr2O3 is amphoteric and Mn2O3 is a completely basic oxide.
The effect can also be clearly seen in the dissociation constants of the oxoacids of chlorine. The effect is much larger than could be explained by the negative charge being shared among a larger number of oxygen atoms, which would lead to a difference in pKa of log10(¼) = −0.6 between hypochlorous acid and perchloric acid. As the oxidation state of the central chlorine atom increases, more electron density is drawn from the oxygen atoms onto the chlorine, reducing the partial negative charge on the oxygen atoms and increasing the acidity.
Variation of electronegativity with oxidation number
0 件のコメント:
コメントを投稿